Designing North American PCRs from the ground up: Lessons learned from creating the North American PCR Catalog
To date, there has not been one place to find all the PCRs created for products made in North America. As such, we set out to create the North American PCR Catalog for 2 reasons:
- With the increasing demand for product transparency largely in the building products industry, there is an increasing need for a centralized, curated source of PCRs created for North America;
- To inventory and benchmark the state of PCR development in North America.
What we learned:
- There is considerable complexity in the program operator space.
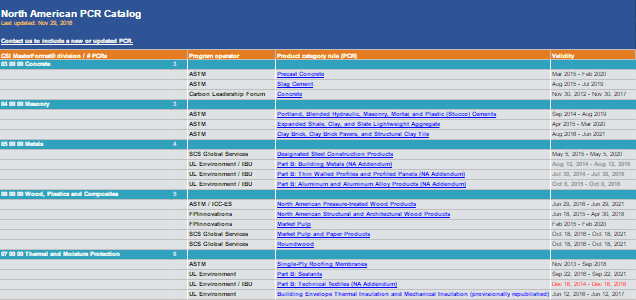
- At the time of publication (09/23/2016), there were 53 PCRs from 8 program operators and programs. 13 had already expired or are due to expire in 2016, 11 of which are addenda to European PCRs. Plus, of the 35 MasterFormat® Divisions, only 15 had PCRs.
- Different types of organizations have become program operators and are making PCRs using different processes and personnel. The organization types include:
- Standards developers and certifiers
- Research companies
- Trade associations
- An industry-academic research collaborative
- A design, technology and innovation company
Each organization makes decisions based on their own expertise resulting in the creation of PCRs that use different technical standards and require different LCA rules.
- PCRs are not comparable.
There are different rules about how each program operates. Read their governance documents. ISO 14025 requires they be publicly available. Often there are different LCA rules used in the same program, depending on the needs of the stakeholder group. This means that the common rules in PCRs from the same program operator may not even be comparable.
- The PCR creation process is unclear.
There is a lack of industry awareness how to get an expiring PCR renewed, how to get a new PCR started, what PCRs are in development and how to participate in the PCR development process.
- Eleven are not PCRs. They are addenda to European PCRs.
The purpose of these addenda is to efficiently create Part Bs for North America (using a 2-part PCR program). Upon review we found:
- Many of these addenda have ‘Draft’ watermarked on each page. (Figure 1)
- Validity date issues
- Addenda have been created for expired PCRs, or the PCR can no longer be found
- The addendum expiration date doesn’t match the addendum expiration statement (Figure 2)
- Nothing indicating involvement of North American interested parties
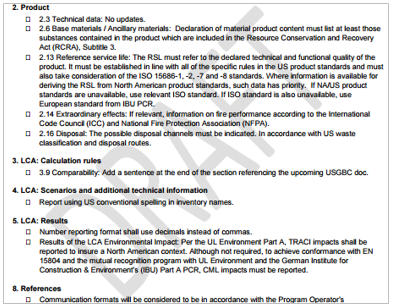
Figure 1. Draft watermark
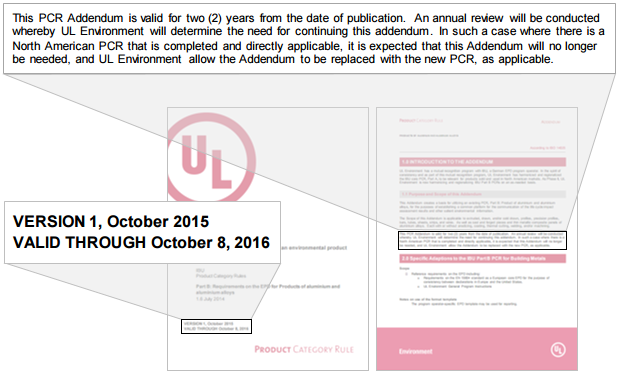
Figure 2. Addendum expiration statement and date
What are we doing about it?
The Sustainable Minds / Program Operator Consortium (SM/POC) Part A has been designed from the ground up for North America. It was designed to support:
- Harmonization with standards and industry
- Standardizing how LCA-based standards for reporting product environmental performance get created
- Providing improved reports to help decision-makers specify materials and products
- Combining efforts to improve access to and recognition of manufacturers’ product transparency reports
The Program Operator Consortium (POC) was formed to provide more useful environmental product transparency solutions and to reduce complexity in the marketplace by standardizing how LCA-based standards for reporting product environmental performance get created, providing improved reports to help decision-makers specify materials and products, and combining efforts to improve access to and recognition of manufacturers’ product transparency reports.
To that end, we all aligned around one common set of LCA calculation rules and reporting requirements – Sustainable Minds’ Part A. What we need is products with actually better environmental performance, not just reports. Aligning to one program that delivers on the intent of ISO 14025 makes the business case for product transparency more compelling. Creating preference for those products by educating the market to understand what the information means will drive behavior change, which means we all benefit!
How is the Consortium solving the market’s problems?
We have a solution for each of the four problems we identified:
- ‘There is considerable complexity in the program operator space.’ Each member of the Consortium follows the same process and follows the same common LCA calculation rules and reporting requirements, no matter who you work with. Learn more >
- ‘PCRs are not comparable.’ Each POC member follows the same common set of rules, as laid out in Sustainable Minds’ Part A. The product group-specific rules then focus the stakeholders on the rules needed only for that product group.
- ‘The PCR creation process is unclear.’ The POC members all follow the same PCR creation process. There is a lot of opportunity to start standardizing now. If there is an expiring PCR in your industry or you’d like to create a new one: Contact us >
- ‘Eleven are not PCRs. They are addenda to European PCRs.’ Since Sustainable Minds’ Part A was built for North America from the ground up, every PCR created using the POC process is custom tailored for North America.
What you can do.
Be a proponent of standardization and simplification. Create new PCRs or renew expiring PCRs with any Consortium member. Become a member. Contact us to get started >